Research Article
Infection Control Mechanisms Employed by Dental Laboratories to Prevent Infection of their Dental Technicians/Technologists

Kilimo C Sammy* and Simiyu N Benjamin
Department of Conservative and Prosthetic Dentistry, School of Dental Sciences, University of Nairobi, Kenya
*Address for Correspondence: Kilimo C. Sammy, Department of Conservative and Prosthetic Dentistry, School of Dental Sciences, University of Nairobi, P.O BOX 19676-00202, Nairobi, Kenya, Tel: +254722869444; E-mail: [email protected]
Dates: Submitted: 14 November 2016; Approved: 28 November 2016; Published: 30 November 2016
How to cite this article: Sammy KC, Benjamin SN. Infection Control Mechanisms Employed by Dental Laboratories to Prevent Infection of their Dental Technicians/Technologists. J Oral Health Craniofac Sci. 2016; 1: 001-011. DOI: 10.29328/journal.johcs.1001001
Copyright License: 2016 Sammy, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Infection Control; Dental Laboratory Technicians; Dental Laboratories; Dental Impressions
ABSTRACT
Objective: The aim of the study was to determine the compliance to infection control of various dental laboratories in Durban.
Study design: This was a qualitative survey.
Setting: Dental laboratories in Durban area, South Africa.
Subject: Registered laboratory technicians.
Study methodology: Convenient random sampling method was used.
Results: There was poor compliance to infection control procedures by most dental laboratories. Majority, 66.67%, of the dental laboratories relied on dental clinics for disinfection of dental impressions; therefore, they did not disinfect the impressions. On the other hand, only 33.33% carried out disinfection of dental impressions on their own. A high number (53.3%) of the respondents had disinfection areas within their dental laboratories, 6.7% had no disinfection areas while 40% depended upon dental clinics for all disinfections. About 60% of the dental technicians had valid vaccinations against Hepatitis B Virus while 40% had no vaccination against HBV.
Conclusion: The results of this study indicated that there was substantial nonconformity to infection control measure in all dental laboratories. There should be comprehensive inspection of dental laboratories prior to licensing and thereafter by the South African Dental Technician Council’s inspectors to ensure that all dental laboratories comply with the various infection control measures.
INTRODUCTION
Infection control is fundamental in a dental laboratory so that dental technicians/technologists can be prevented from getting infected [1]. Before 1970s, infection control was not performed in dental laboratories though there was a major concern on handling of items from “high-risk patients”. It was later realised that microorganisms could survive on saliva and blood and that any patient could be a source of infection. As a result, infection control became apparent and has now resulted in impressive protocols to prevention of disease spread in the dental office and laboratories [1].
Infection control in dental laboratories was first recommended by American Dental Association (ADA) through its recommendations and guidelines of the Centres for Disease Control (CDC). It was published first in 1986 and revised in 1993 [2].
A dental laboratory is an area where dental technicians/technologists can get infected mainly from soiled impressions received from the dental clinics [3,4]. On the other hand, cross infection may arise among dental staff and patients from contaminated items sent from the dental laboratories to dental clinics [5].
It is difficult to disinfect dental casts than impressions because the microorganisms percolate into the inner parts of these casts thus making disinfection arduous. Oral bacteria can remain viable for as long as a week even in set gypsum. Therefore, it is mandatory that all impressions received from the dental surgery are disinfected [6]. Not all infected patients can be diagnosed from their medical histories, physical examinations or readily available laboratory tests. Because of this, Centre for Disease Control (CDC) has introduced what is known as “Universal precautions”. This means that all human blood and saliva should be treated as if known be infectious for Human Immunodeϐiciency Virus (HIV), Hepatitis B Virus (HBV) and other blood borne pathogens [7,8].
Special disinfection area
Disinfecting and working areas should be established. All work and countertops should be cleaned and disinfected on daily basis with an appropriate surface disinfectant and in according to the manufacturer’s directions. In this area, infection control policy must be clearly written and displayed on the wall in form of a poster. The writing must be precise, concise and easy to understand. The policy must be renewed annually or whenever necessary to accommodate new disinfection techniques and eliminate the outdated ones. A provision must be in place for occupational exposure incidents. All incoming cases should be disinfected as soon as they are received and all containers to be sterilized or disinfected after every use. Packing materials should be discarded to avoid contamination [9].
Solid wastes soaked with bloody ϐluids should be put in sealed impervious bags and disposed according to the regulations of the local or national environmental agencies. Technicians/ Technologists working in this area should wear clean laboratory dust coats, face masks, protective eyewear and disposable gloves [10].
Work surfaces and equipment should be kept clean and disinfected daily. All instruments, attachments, and materials to be used on new prostheses should be separated from those used on prostheses that have already been inserted in the mouth. Rag wheels should be washed and autoclaved after every use [11].
Dental laboratory technicians/ technologists may be exposed via direct contact with non-disinfected items such as impressions through cuts and abrasions whenever these impressions are handled without gloves and masks on. In the laboratory, infection can be transferred from cast to the dental technician/ technologist by surface contact, hand-pieces, burs, pumice, aerosols, unwashed hands etc. [12].
The dental laboratory has to be as safe as possible from any kind of infection. This can be ensured by minimizing potential for disease transmission via;
- Immunization- All dental technicians/ technologists must be immunized against hepatitis B virus.
- Barrier Techniques- This include washing hands with antimicrobial soap or an alcohol based hand rub before starting to work in a dental laboratory. The same procedure must be followed after disposing used gloves in readiness for new gloves.While working in the dental laboratory, a dental technician/ technologist should always use personal protective equipment such as gloves, masks, goggles, and lab coats [10]. Utility gloves should be used when cleaning or disinfecting equipment or surface as they are thicker and tougher than the disposable gloves. Masks, protective eyewear or clothing must be used when there is potential for splashes, spray, spatter, or aerosols such as when operating polishing lathes, model trimmers, motors or any other rotary equipment. Laboratory dust coats should be won at all times during fabrication process in the laboratory and be changed or laundered daily and should not be won outside the laboratory. This prevents transmission of contaminants from outside the laboratory to laboratory [12].
A survey by Kugel et al. [13] showed that dentists and laboratory personnel did not communicate well about the disinfection procedures they followed. There should be an essential communication between dental laboratories and dental clinics regarding infection control protocols. This will ensure that appropriate cleaning and disinfection procedures are performed either in the dental office or laboratory so that disinfection is ensured [14].
Disinfection
It is preferred that all disinfection procedures should be done in the dental laboratory by well-trained dental laboratory technician/technologist. Sometimes the disinfection status of an item is unknown. The correct disinfectant should be used to prevent corrosion in metallic components and dimensional changes and surface textures for impressions [7]. Dental laboratories should isolate prostheses of high-risk patients from other laboratory work. When handling these materials, one should wear surgical gloves and mask. All instruments and devices that come into contact with a high- risk patient’s prosthesis must be sterilized [2].
The duration and method of disinfection depends on the potential of the impression material to absorb water and the time lapse between impression-taking and disinfection. Condensation silicones, addition silicones, and polysulphides should be disinfected with disinfectants that do not cause dimensional changes for adequate times or as per the manufacturer’s instruction. After the impression is removed, it should be poured as soon as possible. Long immersion time of hydrophilic impressions causes leaching out of surfactant in the hydrophilic vinyl polysiloxane thus rendering the material less hydrophilic [15]. Polyethers are prone to dimensional changes [16] when immersed for more than 10 minutes. Due to the hydrophilic nature, the best disinfectant for most elastomers is 2% glutaraldehyde. It should be sprayed on the impression until it is saturated then it is wrapped in a towel soaked with disinfectant, then put in a sealed plastic bag for 10 minutes. The paper is removed and the impression is rinsed and poured as soon as possible with gypsum or any other cast material [17].
Methods of disinfection
For irreversible hydrocolloids (Alginate), the recommended disinfectant is chlorine compounds or iodophors using immersion method for less than 10 minutes. For reversible hydrocolloids:
- Polysulphide silicone: Immerse in glutaraldehyde, chlorine compound, iodophors or phenolics for not more than 30 minutes.
- Polyether: Immerse with caution in chlorine compounds or iodophors for less than 10 minutes.
- Zinc oxide eugenol impression paste: Immerse in glutaraldehyde or iodophors.
- Impression compound: Iodophors or chlorine compounds or phenolic spray[17].
Pumice must be changed after the completion of every case. At minimum the pumice and rag wheels should be disinfected daily. The South African compensation for occupational injuries and disease act no. 130 of 1993 states that an employee has a right of compensation (by employer) for injuries and illnesses he/she suffers while working. One can demand for more compensation if it is proved that the employer did not provide enough protective equipment or the infection or if the illness was due to negligence of the employer [18]. Therefore, dental laboratory owners should adhere to all protective, safety, and infection control protocols to avoid meeting the costs for teatments and compensation demands by their employees. It’s thus imperative that infection control practice must cover all aspects of dental activities [6]. The aim of this study therefore, was to determine the compliance to infection control of various dental laboratories in Durban, South Africa.
MATERIALS AND METHODS
This was a qualitative survey using infection control related questionnaires to assess the compliant rates of various dental laboratories in Durban, South Africa. Convenient random sampling was used. The study population were the owners of dental laboratories in Durban area. The study sample included 15 owners of dental laboratories registered with the South African Dental Technicians’ Council. They out to have understood the objectives of the study and the dental laboratories out to have been in operation for at least a year. The questionnaires were designed such that the participants (dental laboratory owner(s) could answer them independently. There were also provisions for additional information and or suggestions related to infection control. A detailed explanation of the background to the study was made along with emphasis on the anonymity of the questionnaire. The returned questionnaires were reviewed for completeness.
Statistical analysis was conducted using the SPSS program (SPSS 16.0 for windows, SPSS Inc., Chicago, USA). All statistical analyses were carried out at a signiϐicance level of 0.05. The collected data were analysed by descriptive statistics and presented in frequency tables. Results were compared by means of cross-tabulation and statistical association tests.
RESULTS
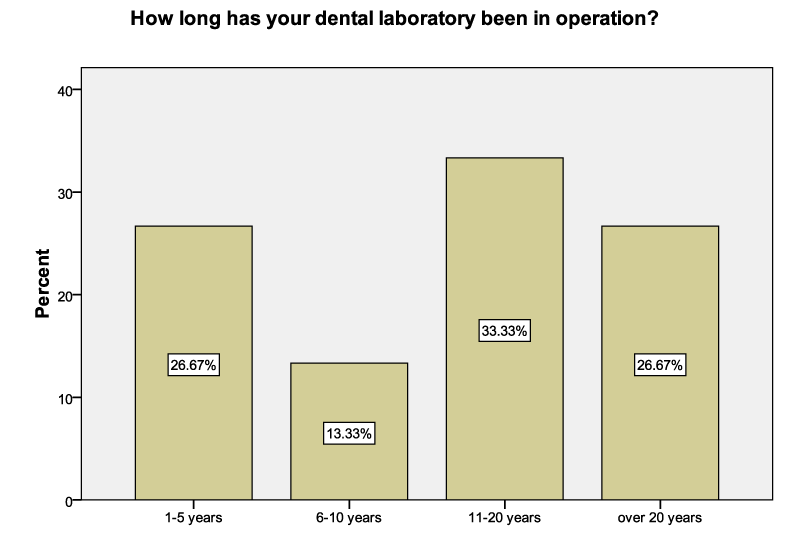
The results were obtained from all the 15 participants (dental laboratory owners) and were interpreted using the various descriptive formats. Sixty Percent (60%) of the laboratories had been in existence for more than 10 years. More than a quarter (26.67%) had been in operation for less than 5 years. Laboratories that had been in existence between 6 to 10 years comprised 13.33% of the sample (Figure 1).
Among the respondents who had speciϐic areas for infection control in their laboratories, only 6.7% had undertaken refresher courses on infection control the previous year. Forty percent of the respondents indicated that it was not applicable for them to have a dental laboratory disinfection area (Table 1).
| Table 1: Cross-tabulation showing areas meant for disinfection only and technicians who had underwent refresher courses. | |||||
| Have you or any of your dental technicians undergone any refresher course on infection control for the past year? | Total | ||||
| yes | no | ||||
| Do you have a specific area in your dental laboratory that is meant for disinfection only? | Yes | Count | 1 | 7 | 8 |
| % of Total | 6.7% | 46.7% | 53.3% | ||
| No | Count | 0 | 1 | 1 | |
| % of Total | 0.0% | 6.7% | 6.7% | ||
| not applicable | Count | 0 | 6 | 6 | |
| % of Total | 0.0% | 40.0% | 40.0% | ||
| Total | Count | 1 | 14 | 15 | |
| % of Total | 6.7% | 93.3% | 100.0% | ||
Nearly one third of the respondents (27%) recruited in this study were employers in dental laboratories that had been in existence for more than 10 years. The crosstabulation below indicates the laboratories by length in existence (Table 2).
| Table 2: Length of existence of Dental Laboratories. | ||||||
| Do you have a specific area in your dental laboratory that is meant for disinfection only? | Total | |||||
| yes | no | not applicable | ||||
| How long has your dental laboratory been in operation? | 1-5 years | Count | 1 | 1 | 2 | 4 |
| % of Total | 6.7% | 6.7% | 13.3% | 26.7% | ||
| 6-10 years | Count | 2 | 0 | 0 | 2 | |
| % of Total | 13.3% | .0% | .0% | 13.3% | ||
| 11-20 years | Count | 2 | 0 | 3 | 5 | |
| % of Total | 13.3% | .0% | 20.0% | 33.3% | ||
| over 20 years | Count | 3 | 0 | 1 | 4 | |
| % of Total | 20.0% | .0% | 6.7% | 26.7% | ||
| Total | Count | 8 | 1 | 6 | 15 | |
| % of Total | 53.3% | 6.7% | 40.0% | 100.0% | ||
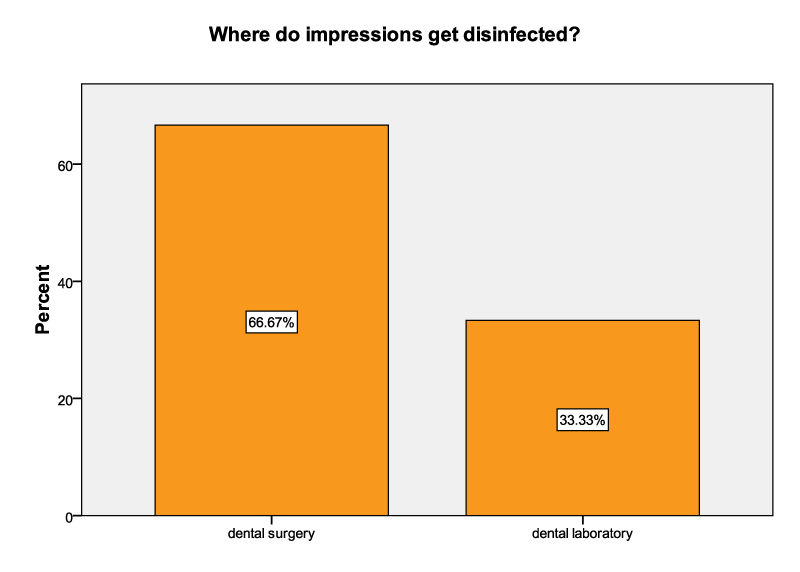
Two-thirds (66.67%) of the correspondents indicated that dental impressions were disinfected at the dental clinic whereas the remaining third indicated that disinfection was done at dental laboratories (Figure 2).
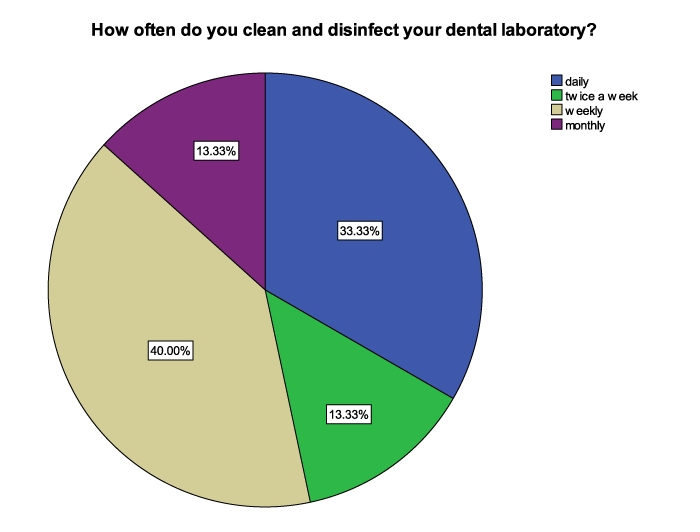
When asked about the compliance of dental technicians/technologists to vaccination against hepatitis B virus, 60% of the respondents had been vaccinated against hepatitis B while 40% percent had no vaccination record for Hepatitis B. When asked about the frequency of disinfection, 33.3% of the respondents indicated that they cleaned and disinfected their dental laboratories daily, 13.33% cleaned and disinfected twice a week, 40.00% cleaned and disinfected on a weekly basis while the remaining 13.33% cleaned and disinfected monthly (Figure 3).
The 13.3% of dental laboratories that were cleaned once a month had been in existence between 6 to 20 years (Table 3). The general trend observed was that cleaning and disinfection was done by dental laboratories that had been in existence for some time, but not as often as it is required.
| Table 3: Correlation between the length of existence and the frequencies of cleaning and disinfecting of the dental laboratories. | |||||||
| How often do you clean and disinfect your dental laboratory? | Total | ||||||
| daily | twice a week | weekly | monthly | ||||
| How long has your dental laboratory been in operation? | 1-5 years | Count | 1 | 0 | 3 | 0 | 4 |
| % of Total | 6.7% | .0% | 20.0% | .0% | 26.7% | ||
| 6-10 years | Count | 0 | 1 | 0 | 1 | 2 | |
| % of Total | .0% | 6.7% | .0% | 6.7% | 13.3% | ||
| 11-20 years | Count | 2 | 0 | 2 | 1 | 5 | |
| % of Total | 13.3% | .0% | 13.3% | 6.7% | 33.3% | ||
| over 20 years | Count | 2 | 1 | 1 | 0 | 4 | |
| % of Total | 13.3% | 6.7% | 6.7% | .0% | 26.7% | ||
| Total | Count | 5 | 2 | 6 | 2 | 15 | |
| % of Total | 33.3% | 13.3% | 40.0% | 13.3% | 100.0% | ||
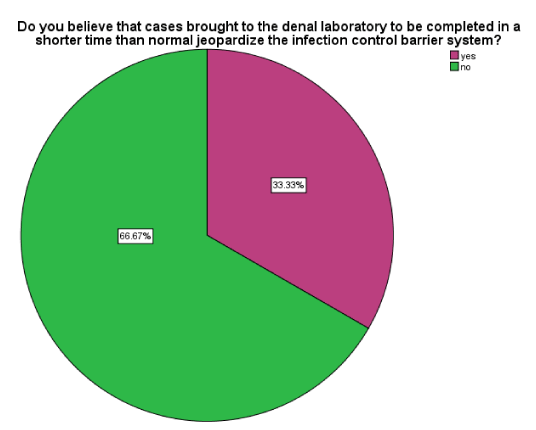
There was a 2:1 ratio of no: yes responses regarding the time period in which laboratory cases were completed. There were 66.67% of the respondents who did not believe that completing laboratory cases (work) in a shorter time greatly affected the infection control barrier systems (Figure 4).
Figure 4 : A pie chart showing the Yes and No responses regarding rush cases that could jeopardize infection control barrier system.
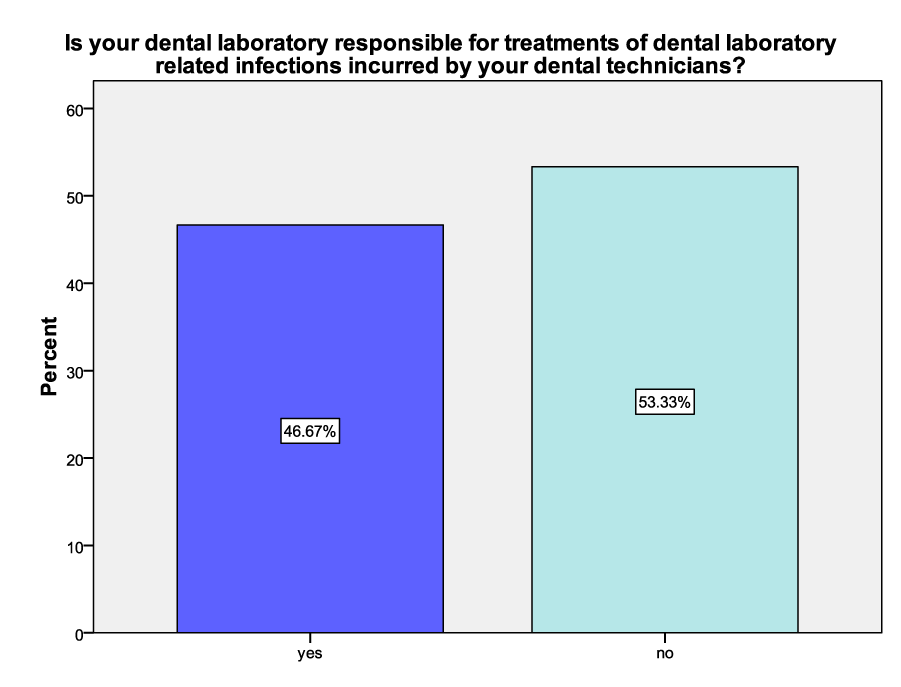
Among the correspondents, 53.33% of the employers were not responsible for the treatment of dental laboratory-related infections incurred by their dental technicians while 46.67% would incur the cost of treatment of such infections (Figure 5).
Figure 5 : A graph showing the percentages of responsibility of dental laboratory employers for treatment costs of dental laboratory-related ilnesses incurred by their dental technicians
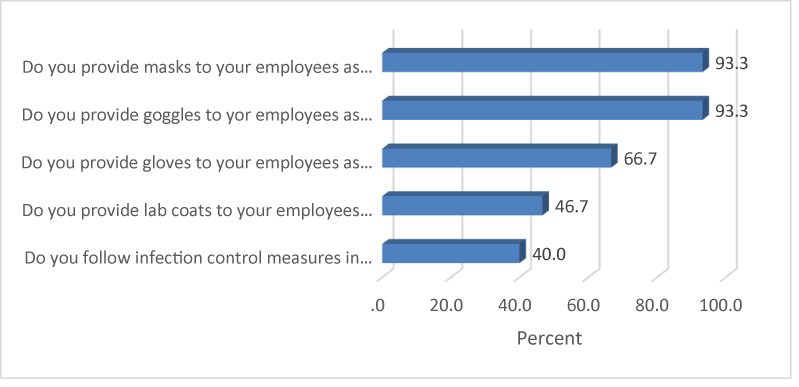
The most common forms of protective gear provided to employees were masks and goggles (each 93.3%). Two thirds (66.7%) of the respondents indicated that gloves were also provided. Slightly less than half (46.7%) of the respondents were in laboratories that issued laboratory dust coats. Only 40% of the laboratories followed infection control measures (Figure 6).
Positive correlations indicated that there was a directly proportional relationship between the variables. The second set of statements had a correlation of 0.557. This means that more cleaning and disinfection took place when laboratories had a dedicated disinfection area. The other sets of results had negative correlations (Table 4). This implied an inverse relationship. For example, for the third set of statements, the less often laboratories were cleaned, the more likely the laboratory would be responsible for the treatment of dental laboratory-related infections incurred by their technicians (r = -0.587). There was a signiϐicant relationship between following infection control measures and having a speciϐic disinfection area in the laboratory (P values =0.025).
| Table 4: The various correlations. | ||
| Statement 1 | Statement 2 | Correlation |
| Where do your impressions get disinfected? | Do you have a specific area in your dental laboratory that is meant for disinfection only? | -.645** |
| Do you have a specific area in your dental laboratory that is meant for disinfection only? | How often do you clean and disinfect your dental laboratory? | .557* |
| How often do you clean and disinfect your dental laboratory? | Is your dental laboratory responsible for treatments of dental laboratory related infections incurred by your dental technicians? | -.587* |
DISCUSSION
Currently, in the context of universal precaution, it is imperative to consider impressions and stones as an eminent risk of contamination [19]. The results of the present study indicated that there was poor compliance to infection control procedures by most dental laboratories. This is because none of the 15 dental laboratories had 100% compliance to infection control. This could be as a result of inadequate knowledge on infection control by most dental laboratory owners. This ϐinding is in concurrent with a study in the United States which indicated a great deal of mystery surrounding impression disinfection techniques used by dental laboratories [13].
There were 66.67% of the dental laboratories that relied on dental clinics for the disinfection of the impressions brought to their dental laboratories; therefore, they did not disinfect impressions. This could be as a result of lack of proper communication between dental clinics and these dental laboratories. The remaining 33.33% carried out disinfection of all impressions in their dental laboratories. This is in contrast with a study by Nabila et al. who recorded that more than 84.00% of the technicians personally disinfected their impressions [20]. This study, therefore shows poor communication between dentists and the dental laboratories which is similar to Kugel et al. [13] who concluded that almost half of the laboratory directors received inadequate instruction in regard to disinfection techniques. For the purposes of avoiding confusion whether an impression had been disinfected or not, as well as prevention of duplication of services, the dental laboratories should essentially communicate regarding the status of every item that is send out of their respective dental clinics. A written communication should be tagged on every impression or prosthesis indicating that it has been disinfected with a speciϐic disinfectant for a certain period. The absence of such a tag should imply that the item was not disinfected.
A high number (53.3%) of the respondents had disinfection areas within their dental laboratories, 6.7% had no disinfection areas and 40% depended on the dental clinics for all disinfections. This ϐinding could be as a result of lack of proper guidelines on handling of dent l impressions/specimens. For the dental laboratories that carried out disinfection despite not having special disinfection areas, disinfection was performed in the dental plaster rooms. This is considered an eminent risk because it can result incross-infection between newly received impressions and the disinfected ones.
Concerning the use of personal protective equipment, there was 93.3% compliance use of goggles and masks and 66.7% and 46.7% for gloves and dust coats respectively. These ϐigures are higher than what was found in previous studies in which 35.00% [21] and 8.70% [20] of the technicians put on all the recommended protective gear. This could mean that dental technicians were aware of personal protective equipment. For example, face masks prevent inhalation of aerosols whose particle sizes could be as small as 50 microns. Furthermore, the use of lab coats and gloves are equally important because they prevent cross-contamination.
About 60% of the dental technicians who shared in this study said that they had valid vaccinations against hepatitis B virus; this ϐinding is almost similar to a study done in Saudi Arabia (63.00%) [20], but higher than former studies where just 10.00% [21] and 24.4% [22] of the technicians had received HBV vaccination. This means that majority of the dental technicians in Durban had received sensitization about HBV, a virus which is spread through contact with blood or certain body ϐluids of an infected person. On the other hand, 40% of the dental technicians had no record of HBV vaccination. This is a purpose for interest, because it means that this group of dental technicians are disposed to infection with HBV. Furthermore, they did not use any protective and safety measures while working. This ϐigure is higher compared to a study done in Saudi Arabia where 17.39% of the technicians didn’t receive Vaccination for HBV [20].
Centre for Disease Control and Prevention (CDC) suggested that work surfaces and equipment should be cleaned and decontaminated with a suitable liquid chemical germicide following accomplishment of work activities [23,24]. From the results of this study, 33.33% of the respondents cleaned and disinfected their dental laboratories daily, 13.33% did it twice a week and monthly while 40% cleaned and disinfected weekly. This is a worrying practice that renders the rest of the precautions useless because the already disinfected items could be re-infected by the microorganisms, which can be viable for as long as seven days on surfaces as well as in the atmosphere. For full compliance to disinfection procedures, dental laboratories should be cleaned and disinfected daily.
Many (93.3%) of the respondents had not undergone any refresher course/training on infection control the previous year. Only one respondent (6.7%) indicated that he had undergone such training. This could mean that either there were no refresher courses on infection control at that time or the available courses were costly. Lack of time to enrol for refresher courses could also have contributed to the low number of technicians who had undertaken refresher courses.
Laboratory work which is brought to the dental laboratory to be completed within a short time, otherwise known as ‘rush cases’, is said to jeopardize the infection control barrier system. There were 66.67% of the respondents who indicated that rush cases did not affect the adherence to the infection control barrier system while 33.33% of the respondents believed that rush cases jeopardized the infection control barrier system. This implies paucity of knowledge regarding rush cases among dental technicians.
Majority, 53.33%, of the respondents said that they were not responsible for the treatment costs of dental laboratory-related infections incurred by their dental technicians/technologists while the remaining 46.67% acknowledged the responsibility. This is contrary to the conditions of employment in accordance with South African Dental Technicians Act 17 of 1979 which states that it is the responsibility of the employer to meet the treatment costs of their employees [18]. This could mean non-compliance to the Act and there for this calls for sensitization on the 1979 Act.
CONCLUSION
The results of this study indicated that there was substantial nonconformity to infection control measure in all dental laboratories. There should be thorough inspection of the dental laboratories prior to licensing and thereafter by the South African Dental Technician Council’s inspectorate to ensure that all dental laboratories comply with the various infection control measures.
RECOMMENDATIONS
Mandatory infection control continuing education courses and seminars should be conducted to update the dental technicians on the current infection control protocols. This will expand conformity to infection control principles. This research can be used as a source for additional multi-centre studies to reveal nonconformities with all suggested infection control programs by dental technicians as well as communication between dental technicians and dental clinics.
ACKNOWLEDGMENT
The Authors wish to acknowledge the following people for their guidance and enthusiasm: Mr. G. B. Somers for his guidance throughout the duration of this study. Mr D. Singh for his statistical guidance. The Durban University of Technology (DUT) for allowing us to use library and computer laboratory services.
REFERENCES
- Miller CH. Infection control. Dent Clin North Am. 1996; 40: 437-456. Ref.: https://goo.gl/cFmEUm
- Cottone JA, Terezhalmy GT, Molinari JA. Practical Infection Control in Dentistry. 2nd edition. USA. 1996; Williams &Wilkins. Ref.: https://goo.gl/nexrVc
- Verran J, Kossar S, McCord JF. Microbiological study of selected risk areas in dental technology laboratories. J Dent. 1996; 24: 77-80. Ref.: https://goo.gl/oOZBkZ
- Dental Laboratory Relationship Working Group of the Organization for Safety and Asepsis Procedures (OSAP). OSAP Position Paper: Laboratory Asepsis. 1998. Ref.: https://goo.gl/HolAzn
- Infection control recommendations for the dental office and the dental laboratory. Council on Dental Materials, Instruments, and Equipment. Council on Dental Practice. Council on Dental Therapeutics. J Am Dent Assoc. 1998; 116: 241-248. Ref.: https://goo.gl/Mw3ZPM
- Al-Kheraif AA, Mobarak FA. Infection control practice in private dental laboratories in Riyadh. Saudi Dental Journal. 2008; 20: 163-169. Ref.: https://goo.gl/WjTYmb
- http://www.cdc.gov. Accessed on 01/09/2010.
- Porter SR, Peake G, Scully C, Samaranayake LP. Attitudes to cross-infection measures of UK and Hong Kong patients. Br Dent J. 1993; 175: 254-257. Ref.: https://goo.gl/RhQFxY
- Twomey JO, Abdelaziz KM, Combe EC, Anderson DL. Calcium hypochlorite as a disinfect- ing additive for dental stone. J Prosthet Dent. 2003; 90: 282-288. Ref.: https://goo.gl/dzlw3P
- USAF Guidelines for Infection Control in Dentistry, September 2004. Available from: http://www.brooks.af.mil/dis/infcontrol.htm.
- http://moderndentalus.com. Accessed on 01/08/2016.
- CDC Guidelines for infection control in dental health-care settings-2003. MMWR 2003. 52 (No. RR-17). Ref.: https://goo.gl/BBOzr7
- Kugel G, Perry RD, Ferrari M, Lalicata P. Disinfection and communication practices: a survey of U. S.dental laboratories. J Am Dent Assoc. 2000; 131: 786-792. Ref.: https://goo.gl/OtrM8g
- Lepe X, Johnson GH, Berg JC. Surface character- istics of polyether and addition silicone impression mate- rials after long-term disinfection. J Prosthet Dent. 1995; 74: 181-186.Ref.: https://goo.gl/6DOs9e
- Croser D, Chipping J. Cross Infection Control in General Dental Practice: A practical guide for the whole dental team. London: Quintessence. 1989.
- Johnson GH, Drennon DG, Powell GL. Accuracy of elastomeric impressions disinfec- ted by immersion. J ADA. 1998; 116: 525-530. Ref.: https://goo.gl/ErVG7s
- Anusavice KJ. Philip science of dental materials, 11th Edition. London: Saunders 2003.
- Sudeshni N, Punkaj G. Guidelines for HIV and AIDS Management. Clinical Management and Guidelines for Dentists. Houghton: South African Dental Association (SADA) 2009.
- Ferreira FM, Novais VR, Simamoto PC Jr, Soares CJ, Neto AJF. Evaluation of Knowledge About Dis- infection of Dental Impressions in Several Dental Schools. Rev Odontol Bras Central. 2010; 19:285-289. Ref.: https://goo.gl/gNBMGU
- Sedky NA, Hamid AA, Moazen RE. Study of awareness about infection control procedures in dental laboratories and prosthodontics’ clinics in al-qassim province, kingdom of Saudi Arabia. Egyptian Dental Journal. 2013; 59: 2703. Ref.: https://goo.gl/aofhNh
- Al-Dwairi ZN. Infection control procedures in com- mercial dental laboratories in Jordan. J Dent Educ. 2007; 71: 1223-1227. Ref.: https://goo.gl/X0j7fR
- Akeredolu PA, Sofola OO, Jokomba O. Assess- ment of knowledge and practice of cross--Infection control among Nigerian dental technologists. Niger Postgrad Med J. 2006; 13: 167-171. Ref.: https://goo.gl/BXOJVZ
- Sehulster L, Chinn RY; CDC; HICPAC. Guidelines for environmental infection control in health- care facilities: Recommendations of CDC and the Health- care Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003; 52: 1-42. Ref.: https://goo.gl/1SULLP
- Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, et al. Guidelines for Infection Control in Dental Health-Care Settings—2003. MMWR Recomm Rep. 2016; 52: 1-61. Ref.: https://goo.gl/awqJR4